Search Results
Results for: 'polar bond'
Non-polar compounds - insolubility
By: HWC, Views: 11011
• A non-polar molecule has uniform distribution of electrons. • Non-polar compounds like fatty acids in lipids have a high proportion of carbon and hydrogen. • Lipids possess no charge or partial charge. • Lipids are not attracted to water molecules. • Lipids are not soluble in...
By: HWC, Views: 10415
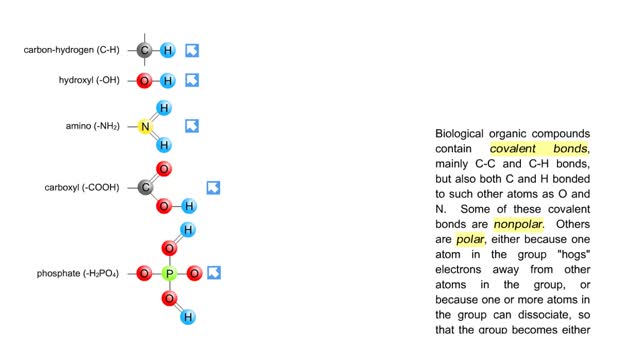
Biological organic compounds contain covalent bonds, mainly C-C and C-H bonds, but also both C and H bonded to such other atoms as O and N. Some of these covalent bonds are nonpolar. Others are polar, either because one atom in the group "hogs" electrons away from other atoms in the group, or...
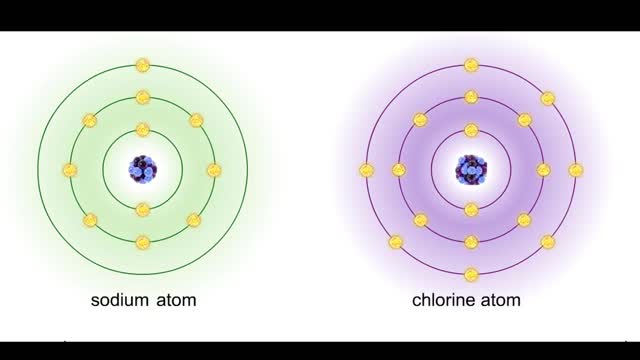
Bond in biological molecules (Ionic, Covalent and Hydrogen bonds)/ How atoms bond?
By: HWC, Views: 8244
Sodium atoms and chloride atoms have unfilled orbitals in their outer shells. The lone electron in the outermost shell of a sodium atom can be pulled or knocked out. This ionizes the atom. It is now a positively charged sodium ion. A chlorine atom has an electron vacancy in its outer shell and...
Properties of water -structure of water and polarity (Ionized and polar compounds)
By: HWC, Views: 11042
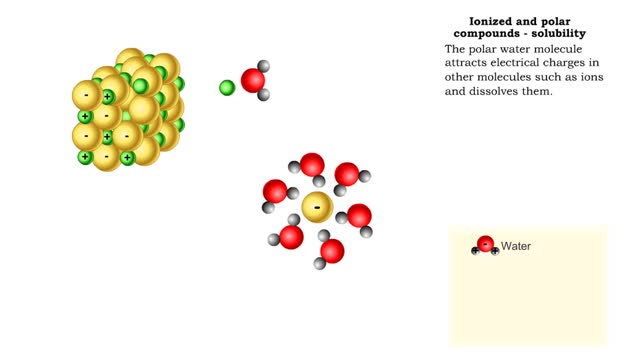
■ Water transports most of the molecules in the body. ■ The structure of a water molecule allows it to dissolve other molecules. ■ Shared electrons spend more time near the oxygen atom. ■ Oxygen end has a partial negative charge. ■ Hydrogen ends have a partial positive charge....
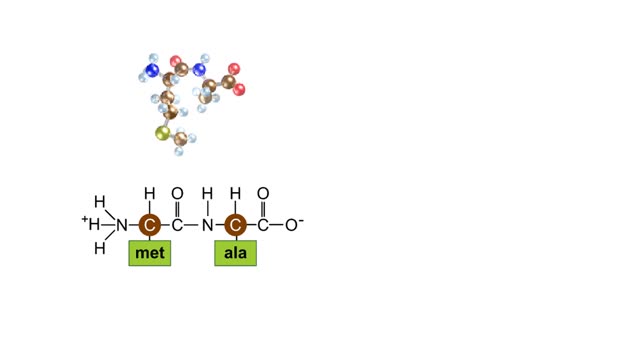
Peptide Bond Formation Animation
By: HWC, Views: 4677
During protein synthesis, peptide bonds link amino acids together in the order specified by DNA instructions. In this case, the first two amino acids in the protein are methionine and alanine. Here are ball-and-stick models of these amino acids. Peptide bond formation is a type of condensatio...
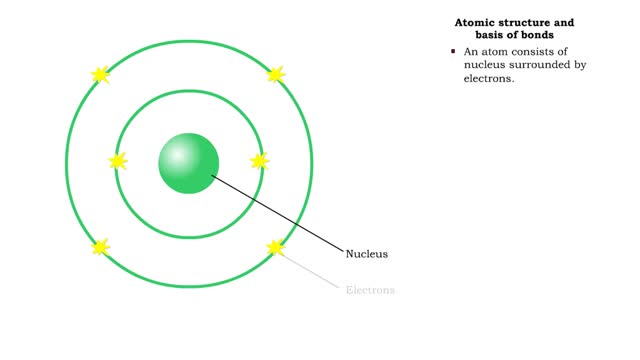
Bond types - Atomic structure and basis of bonds
By: HWC, Views: 11300
• Chemical bonds are fundamental to the structure and function of many types of molecules, such as proteins, carbohydrates, lipids, nucleic acids, gases, salts and water. ■ These molecules are composed of atoms that are held together by three different types of bonds. • The three types ...
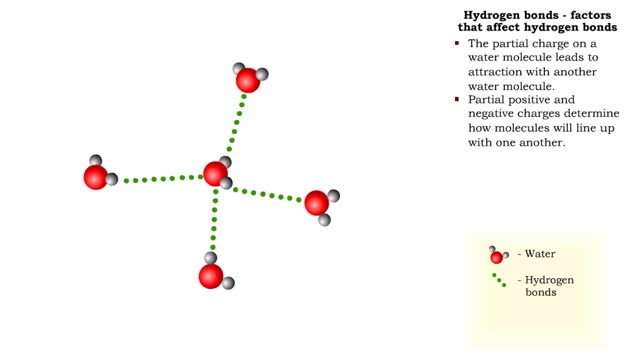
Hydrogen bonds - role in the body
By: HWC, Views: 11211
A hydrogen bond is the electromagnetic attraction between polar molecules in which hydrogen is bound to a larger atom, such as oxygen or nitrogen. This is not a sharing of electrons, as in a covalent bond. Instead, this is an attraction between the positive and negative poles of charged atoms. ...
By: HWC, Views: 9699
The slight positive charge of a hydrogen atom in a water molecule can attract an atom with a slight negative charge, such as the nitrogen in a molecule of ammonia. This forms a hydrogen bond between the two atoms. Hydrogen bonds join the two strands of a DNA molecule. Although hydrogen bo...
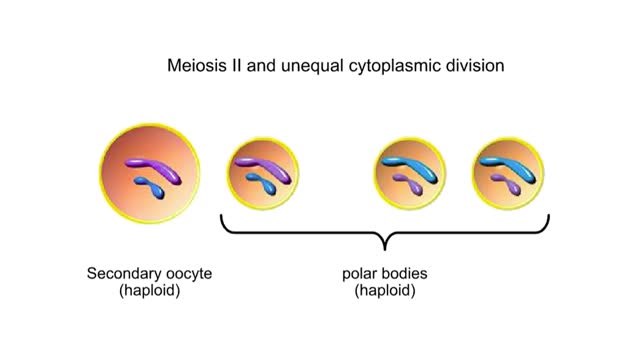
Egg and Sperm Formations in Animals Animation
By: HWC, Views: 5369
Inside the ovaries of a female animal are diploid germ cells called oogonia. An oogonium grows to become a primary oocyte. This large cell is still diploid. Meiosis I followed by unequal cytoplasmic division produces one large secondary oocyte and a smaller polar body. Both are haploid. ...
Advertisement